Noninvasive Treatment for Spinal Disc Disease
Discogen is developing an ultrasound-based treatment of intervertebral discs to provide symptomatic relief of both low back and leg pain. Based on in vitro and in vivo studies, the Discogen system – DiscRx™ – is biologically active in reducing inflammation and promoting spinal disc repair processes.
This proprietary technology will be validated in a First-In-Human study beginning at Columbia University in March 2025.
If you are interested in learning more, or have patients that might benefit, please visit this Columbia RecruitMe page for further details.

Overview
Spinal disc disease affects a large and growing population in the US and in developed countries around the world. A large number of patients in the US who are not yet candidates for surgery suffer from discogenic back pain — an estimated 6 million new patients annually.
These patients are treated with a spectrum of interventions ranging from physical therapy to epidural injections to implanted spinal stimulators. However, these interventions have varying rates of success and do not have any effect on the underlying pathophysiology, which often leads to later surgery.
DiscRx™ is a disruptive technology that is capable of restructuring the treatment programs for discogenic pain. It will offer both symptomatic relief and also affect the disc degeneration process. It will do so at a projected cost that is substantially lower than current prescribed treatments.
Our Technology
DiscRx™ will promote the repair of early-stage disc disease and relieve inflammation, providing symptomatic relief of pain. DiscRx™ therapy will promote the tissue remodeling and repair processes that may slow the progression of spinal disc disease.
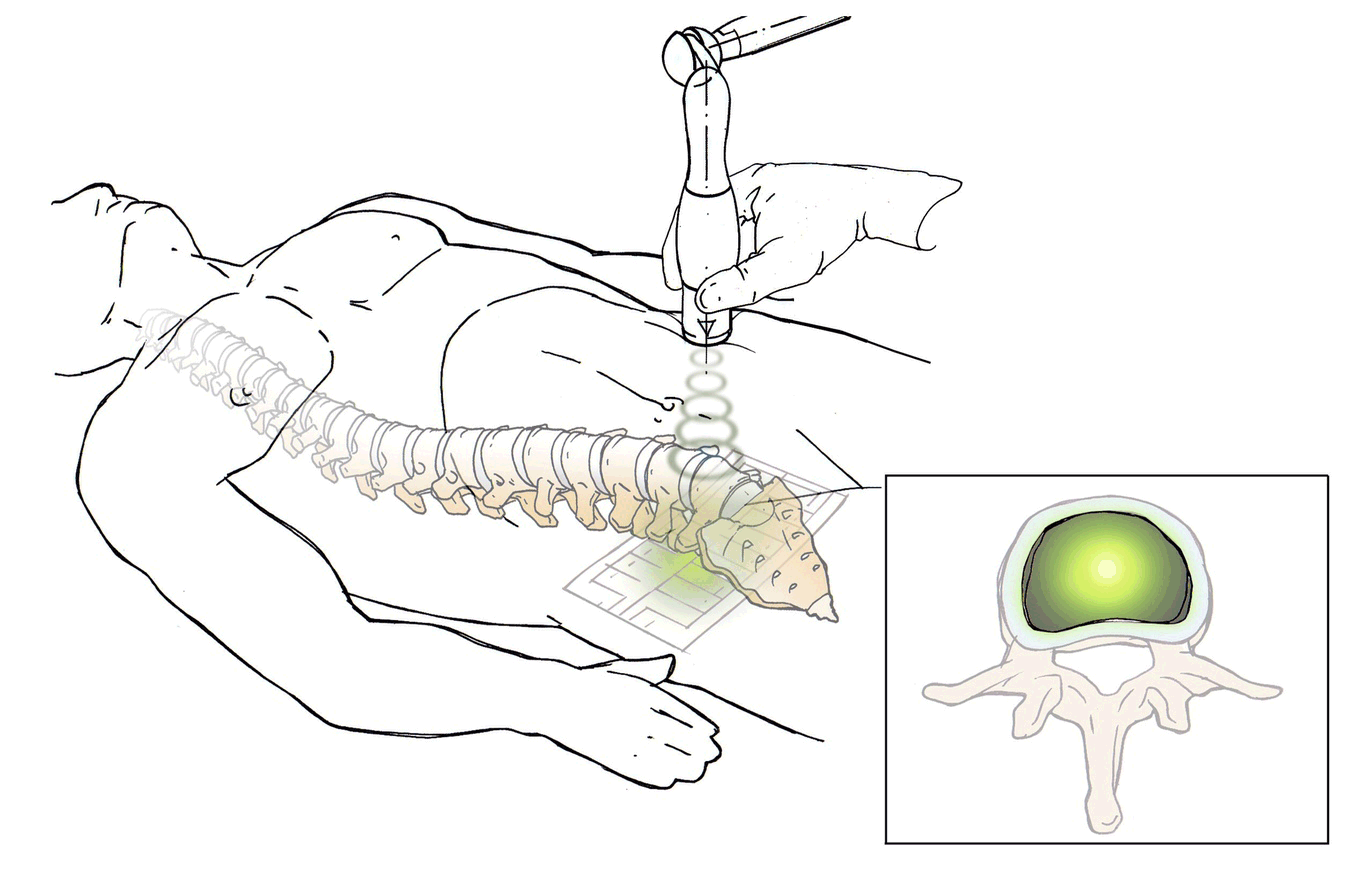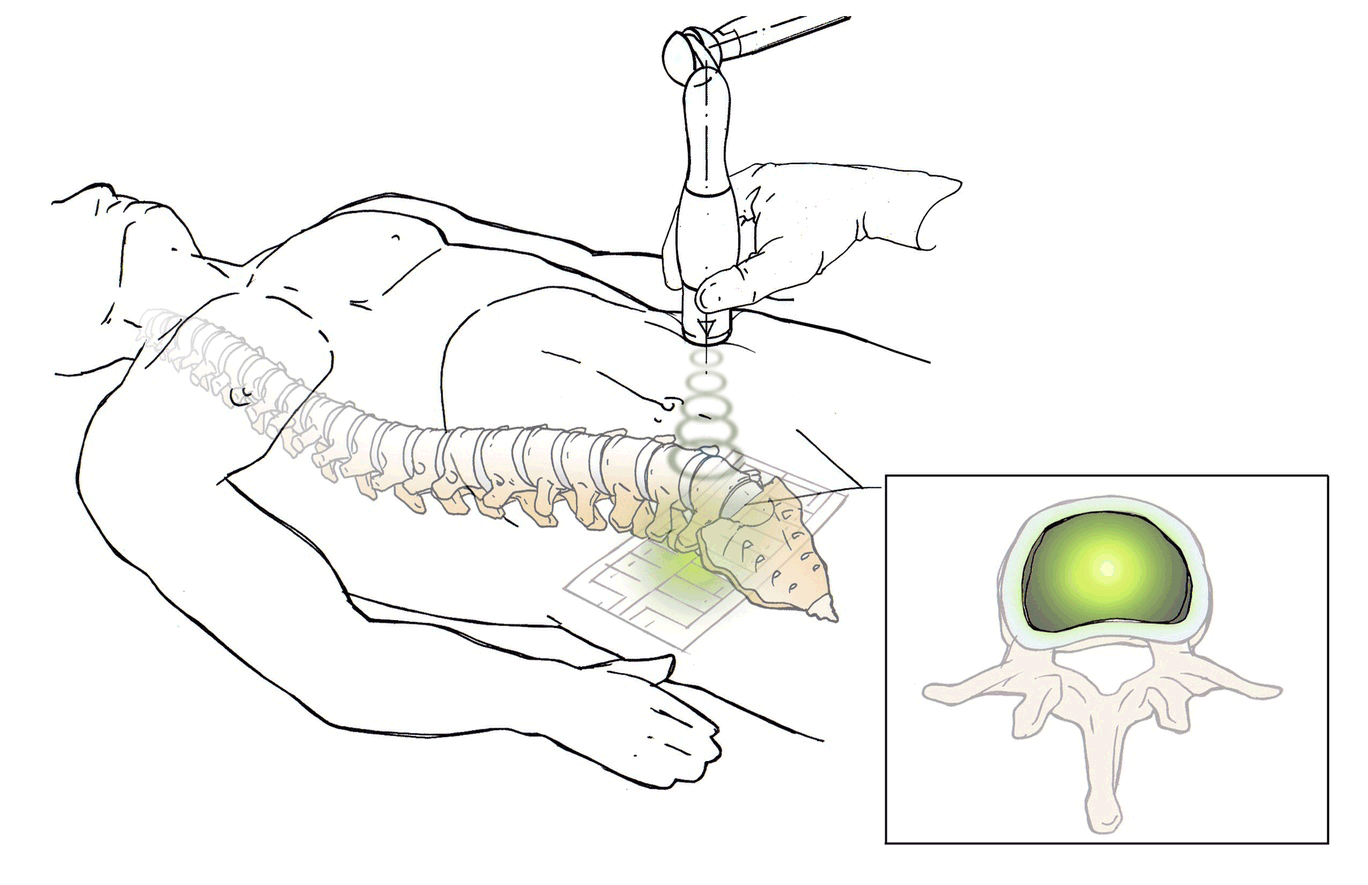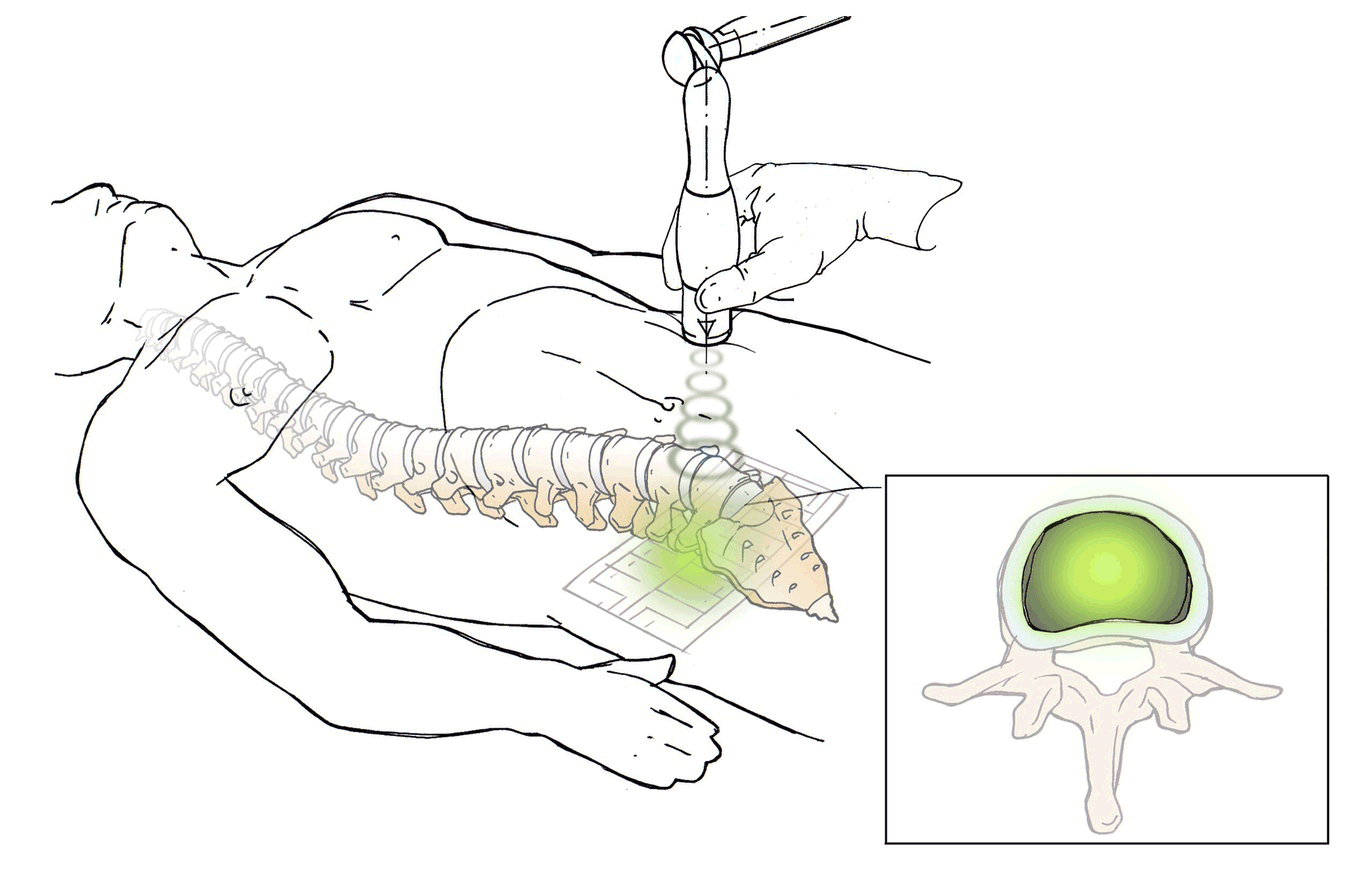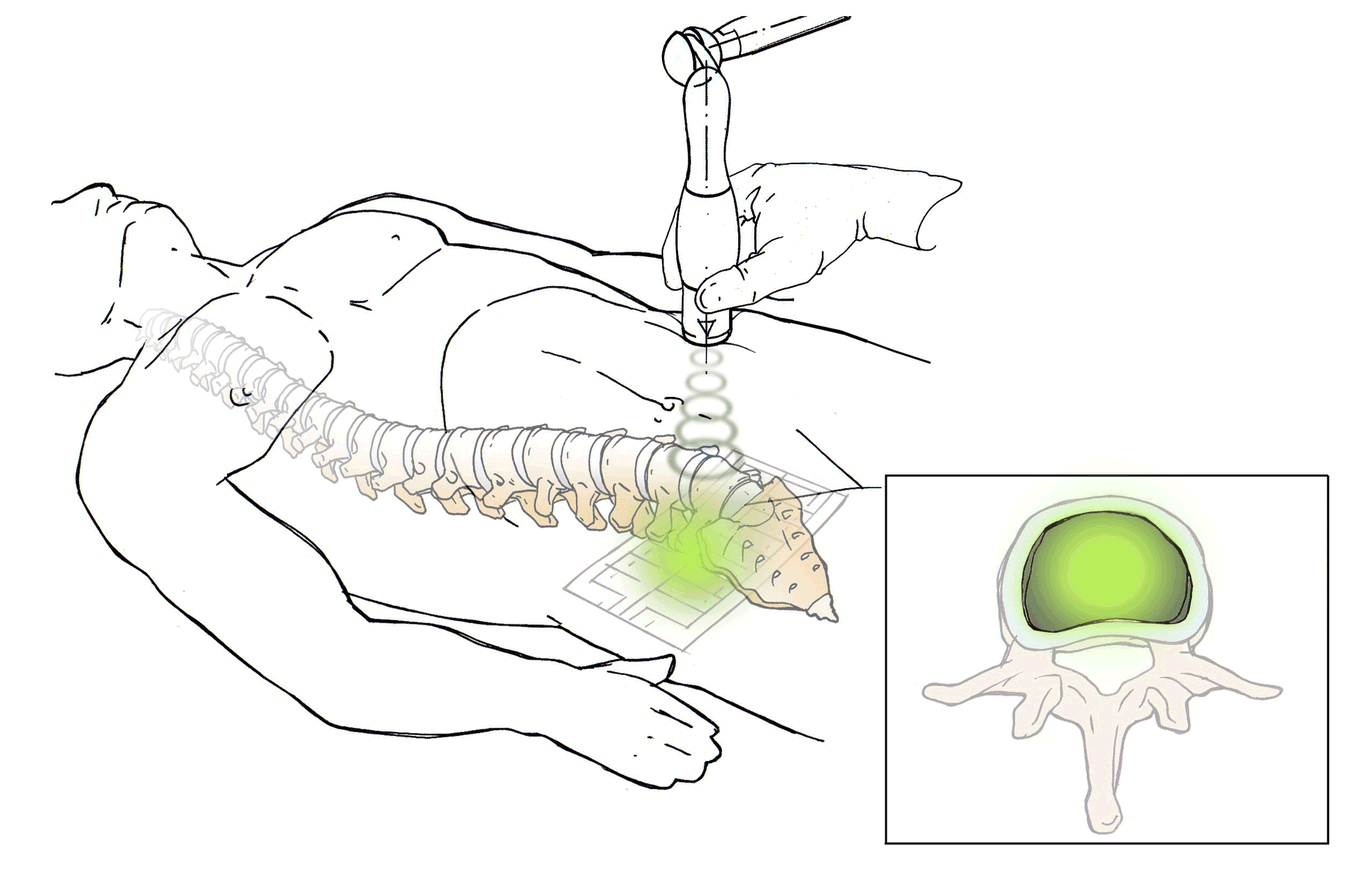
For early- to mid-stage disc degeneration patients, DiscRx™ may delay the time at which surgery is indicated. Delaying surgery by several years has the potential of avoiding additional surgeries as a result of adjacent level disease.
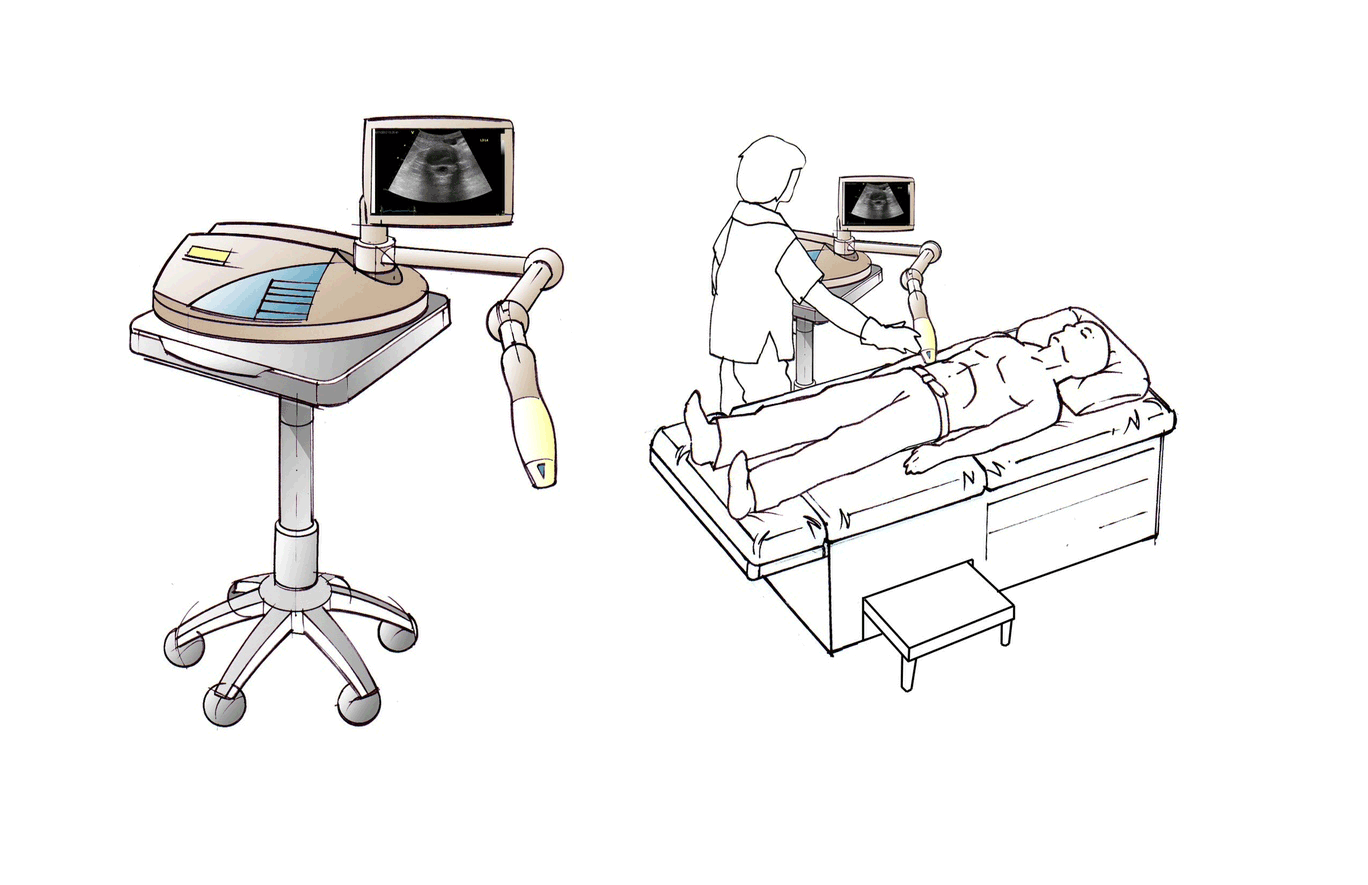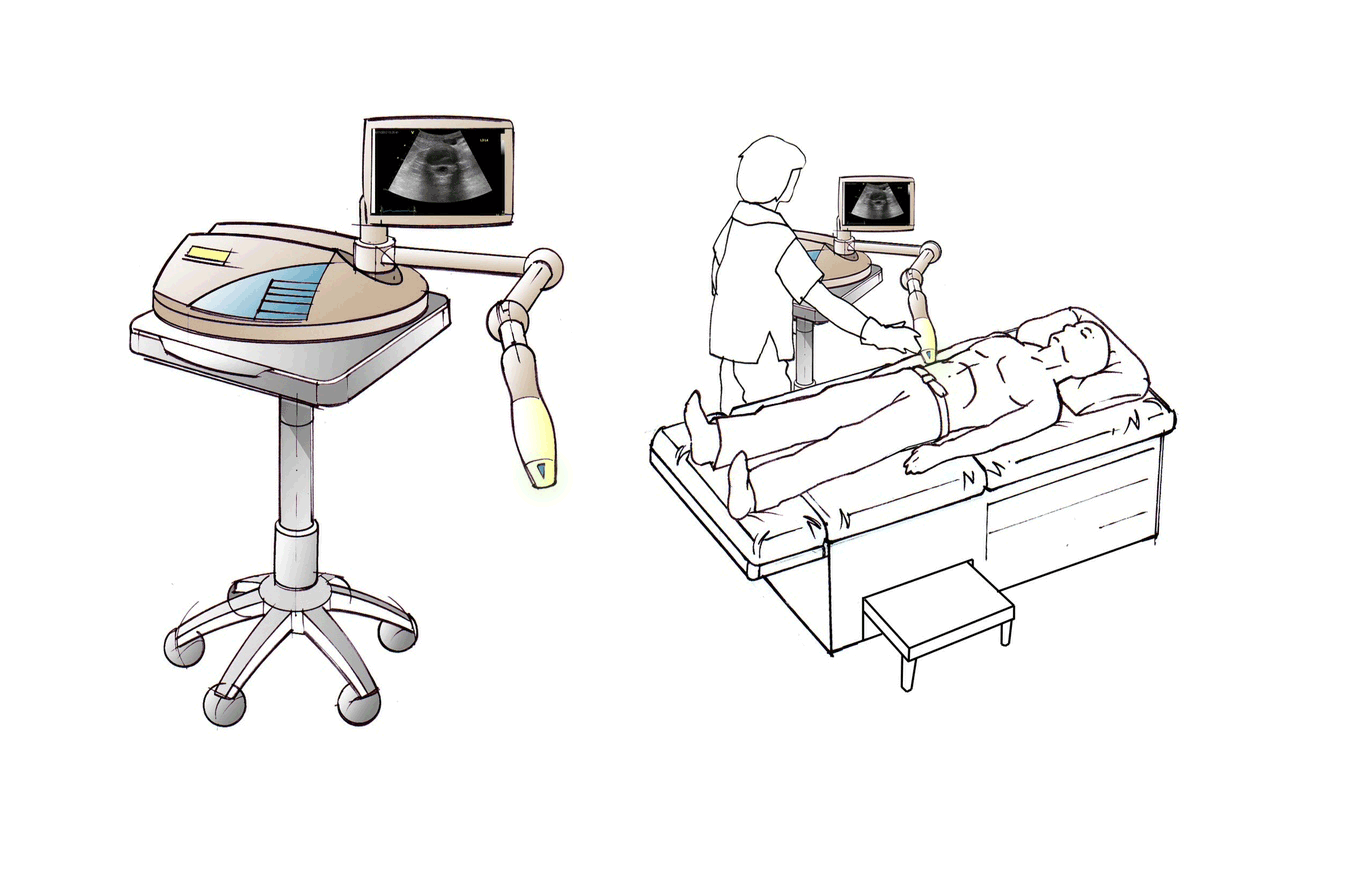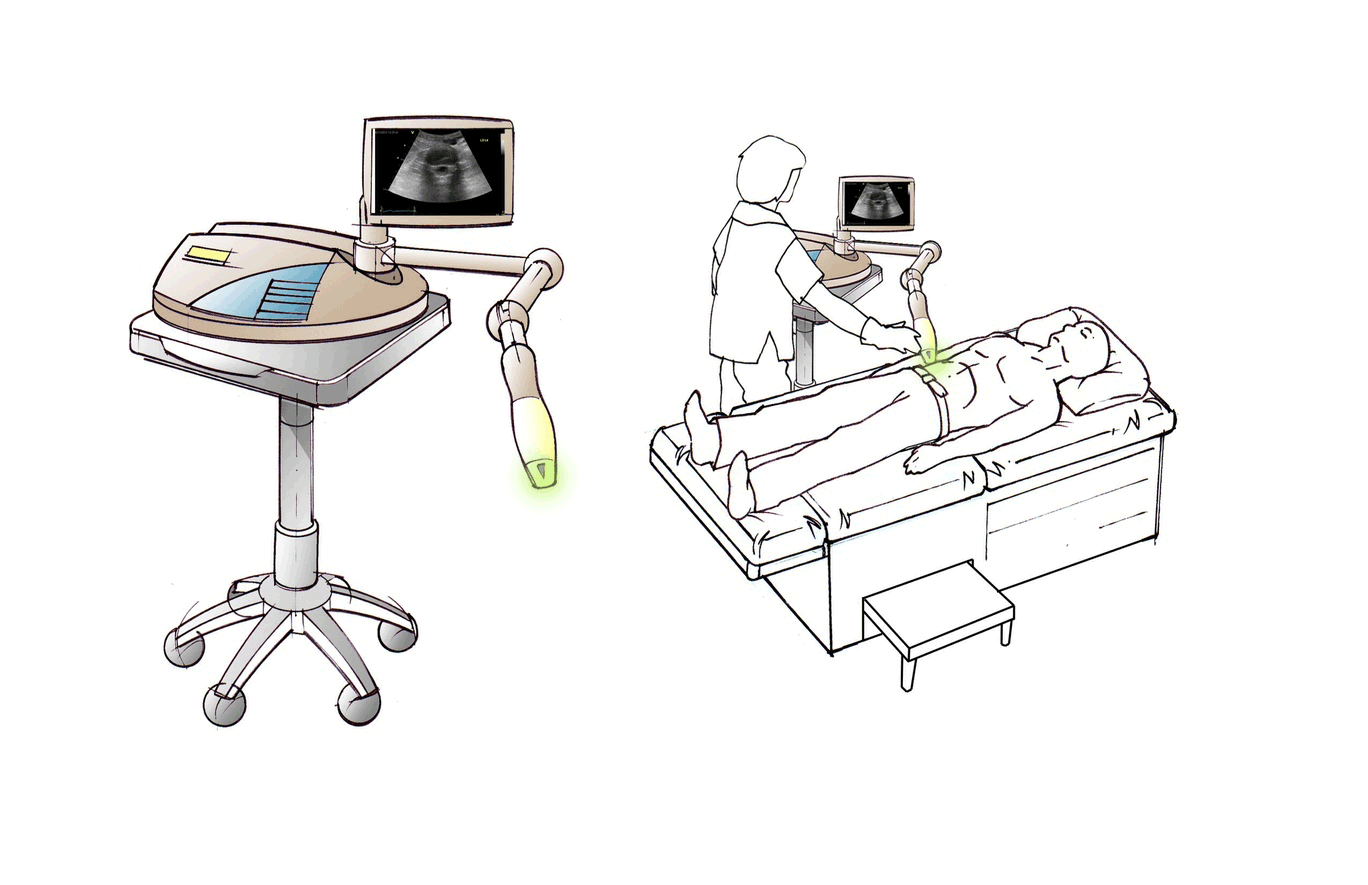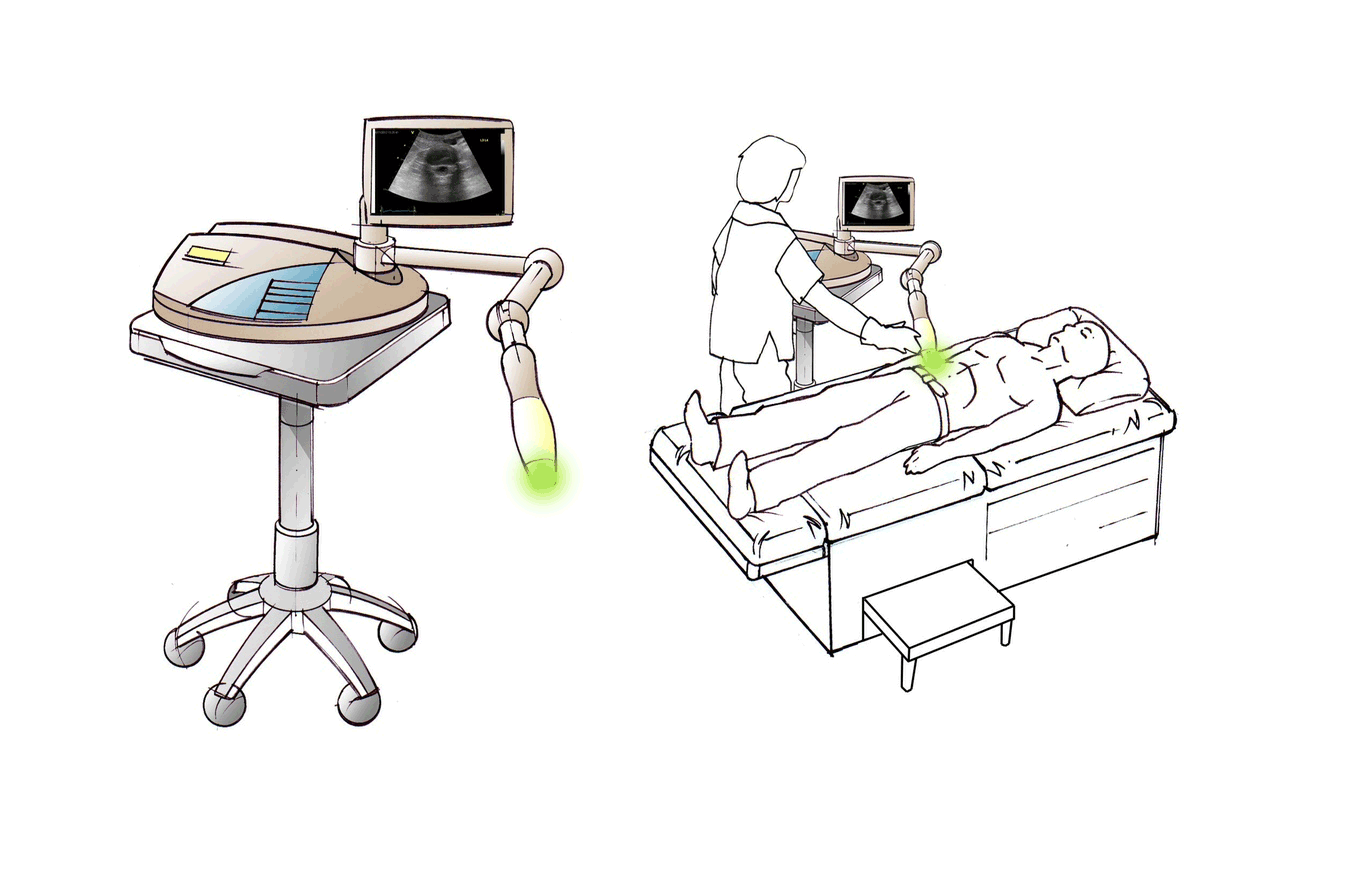
About Us
LEADERSHIP AND ADVISORY BOARD

Peter Zahos
MD, FAANS, FACS. Founder, Chief Executive Officer

Jeffrey Lotz
PhD, Professor, Director, Bioengineering Lab, University of California, San Francisco

Nicholas Theodore
MD, FAANS, FACS. Director, Neurosurgery Spine Program, Johns Hopkins Department of Neurosurgery

Chris J. Diederich
Diederich, Ph.D. Professor, Director, Thermal Therapy Research Group, University of California, San Francisco
About
Discogen is a medical technology development company that designs, patents, and commercializes devices and therapeutics for the Discogenic Back Pain (DBP) market.
Discogen is a medical technology development company that designs, patents, and commercializes devices and therapeutics for the Discogenic Back Pain (DBP) market.